Carbon Capture Update
Note: We at CCL are working hard to speed the transition to clean energy. Getting to NET ZERO (and even to negative emissions to move back from the brink) requires removing CO2 from the air. Here are two projects to keep an eye on.
Note 2: Anyone who’s studied high school chemistry remembers the energy released from the oxidation reaction that includes combustion of hydrocarbons, where the “hydro” joins with oxygen to create water vapor and the “carbon” parts join with oxygen to create CO2. They realize that reversing that process — driving CO2 into some form that can be concentrated and stored — requires a LOT of energy … that is, a lot of cost. It’s even harder because CO2 in the air is so dilute. (Today’s 415 parts-per-million is the same as 415/1,000,000 = 0.04%!)
Note 3: Taking CO2 out of air — called “Direct Air Capture” — is VERY expensive. If you take the more concentrated CO2 out of smokestacks (where the concentrations can be upwards of 20%), it is cheaper, but still > $200/ton of CO2.
Here are the two stories:
Carbon Dioxide Removal Efforts Seen Far Behind What Is Needed
Study finds that engineered approaches for CO2 removal must increase by a factor of at least 1,300 by mid-century.
Photographer: Patrick Pleul/picture alliance/Getty Images
If there’s to be any hope of halting global temperatures from rising more than 2C (3.6F) this century, it will require a mammoth scaling up of carbon dioxide removal (CDR) technologies to vacuum up billions of tons of the stuff from the atmosphere.
But removal efforts to date have largely focused on natural climate solutions like reforestation or storing CO2 in soil. To avoid the worst of a warming planet, engineered approaches that include sucking the emissions directly from the atmosphere must increase by a factor of at least 30 by 2030 and 1,300 by mid-century, researchers from a coalition including the University of Oxford and the German Institute for International and Security Affairs said in a report Thursday.
“We find a gap between how much CDR countries are planning and what is needed in scenarios to meet the Paris temperature goal,’’ the authors wrote. “There are currently few plans by countries to scale CDR above current levels, exposing a substantial shortfall.’’
Of the roughly 2 billion tons of CO2 removed from the atmosphere each year, only about 0.1% currently come from engineered approaches, the report found. Although removal technologies remain controversial — some critics argue the efforts are expensive and serve to extend the life of fossil fuels — one bright spot may be in the US, where supporters say new tax incentives in the US Inflation Reduction Act (IRA) are transformative enough that the technology is finally ready to take off.
Two of the main approaches are carbon capture and storage (CCS) and direct air capture (DAC). The former involves collecting CO2 as it’s being emitted by a big source of pollution like a generator burning fossil fuel to make electricity, while the latter aims to suck emissions directly from the atmosphere. IRA increases the amount of credit from $45 a ton to $85, for CO2 removed from a smokestack, and as much as $180 if the gas is taken from the air.
To be sure, many existing efforts to capture carbon and storage projects it have been beset by problems. Chevron’s Gorgon project, one of the world’s largest carbon sequestration endeavors, has struggled to meet targets to capture and store its own emissions and has in the past had to purchase offsets to address the shortfall.
Despite the number of projects in development growing to a record level last year, they likely only mitigate less than 1% of annual emissions. And even after capturing CO2, projects can face storage challenges and some companies are looking at sticking the greenhouse gases into emptied oil fields.
The authors of the paper argue the technology is crucial to meeting global climate goals and that the amount of carbon removal development required in the second half of the century will only be feasible if there is substantial new deployment in the next 10 years.
“We really need to start deploying these brilliant really novel technologies that are at tiny scale now,’’ one of the authors, Gregory Nemet with the University of Wisconsin–Madison, said in an online briefing. “That needs to happen really quickly.”
============================
Could the ocean hold the key to reducing carbon dioxide in the atmosphere?
UCLA researchers propose strategy that uses seawater to trap billions of tons annually
Wayne Lewis | January 12, 2021
Most experts agree that halting climate change — and the global warming, extreme heat events and stronger storms that come with it — will require the removal of carbon dioxide and other greenhouse gasses from the atmosphere. But with humans pumping out an estimated 37 billion metric tons of carbon dioxide annually, current strategies for capturing it seem likely to fall short.
Now, a UCLA research team has proposed a pathway that could help extract billions of metric tons of carbon dioxide from the atmosphere each year. Instead of directly capturing atmospheric carbon dioxide, the technology would extract it from seawater, enabling the seawater to absorb more. Why? Because, per unit volume, seawater holds nearly 150 times more carbon dioxide than air.
The researchers outline their concept, dubbed single-step carbon sequestration and storage, or sCS2, in a paper published today in the journal ACS Sustainable Chemistry & Engineering.
“To mitigate climate change, we need to remove carbon dioxide from the atmosphere at a level between 10 billion and 20 billion metric tons per year,” said senior author Gaurav Sant, director of the UCLA Institute for Carbon Management and a Samueli Fellow and professor of civil and environmental engineering and of materials science and engineering at the UCLA Samueli School of Engineering. “To fulfill a solution at that scale, we’ve got to draw inspiration from nature.”
Since the atmosphere and the oceans are in a state of equilibrium, if carbon dioxide were to be extracted from the ocean, carbon dioxide from the atmosphere could then dissolve into it. In this scenario, seawater is like a sponge for carbon dioxide that has already absorbed its full capacity, and the sCS2 process aims to wring it out, allowing the sponge to absorb more carbon dioxide from the atmosphere.
The proposed technology would incorporate a flow reactor — a system that continuously is fed raw materials and yields products. The seawater would flow through a mesh that allows an electrical charge to pass into the water, rendering it alkaline. This kicks off a set of chemical reactions that ultimately combines dissolved carbon dioxide with calcium and magnesium native to seawater, producing limestone and magnesite by a process similar to how seashells form. The seawater that flows out would then be depleted of dissolved carbon dioxide and ready to take up more. A co-product of the reaction, besides minerals, is hydrogen, which is a clean fuel.
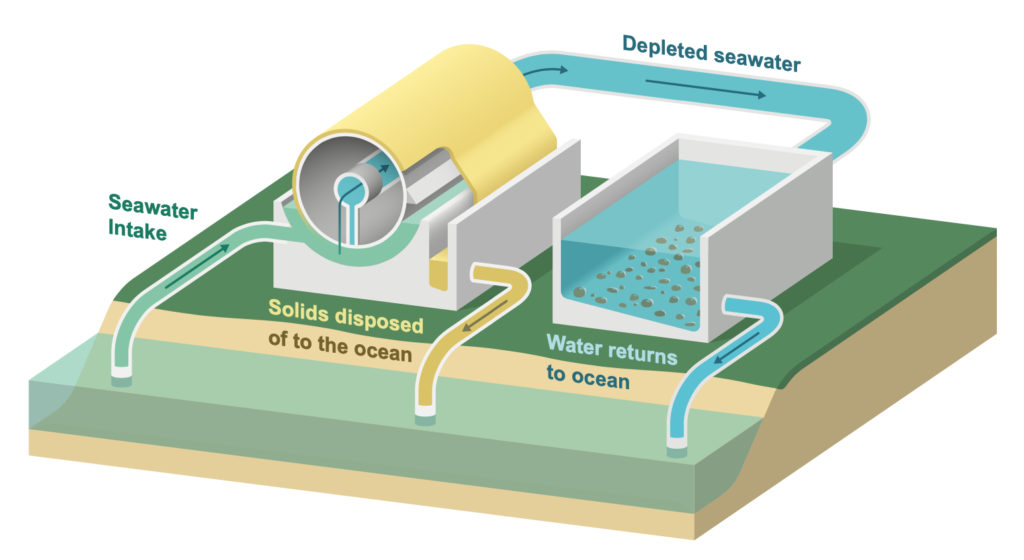
In addition to its potential scale of billions of metric tons, the approach suggested by the UCLA team has important advantages over current ideas for addressing the atmospheric accumulation of carbon dioxide.
The name includes “single-step” to differentiate it from other concepts that require carbon dioxide from the atmosphere to undergo a multistep concentration process before it can be stored. And while some plans propose storing captured carbon dioxide in geological formations such as depleted natural oil and gas reservoirs, there is a risk of leaks returning that carbon dioxide into the atmosphere. By contrast, sCS2 is meant to durably store carbon dioxide in the form of solid minerals.
“What’s nice about turning carbon dioxide into a rock is, it’s not going anywhere,” said Sant, who is a member of the California NanoSystems Institute at UCLA.
“Durable, safe and permanent storage is the premise of our solution,” added first author Erika Callagon La Plante, a former UCLA assistant project scientist who is currently an assistant professor at the University of Texas at Arlington.
The team carried out detailed analyses of the material and energy inputs and the costs required to realize their concept, as well as what to do with the byproducts. Unsurprisingly, given the enormous magnitude of the carbon challenge, they estimate that it would take nearly 1,800 sCS2 plants to immobilize 10 billion metric tons of carbon dioxide each year, with a cost in the trillions of dollars.
“We should be clear: Managing and mitigating carbon dioxide is foremost an economic challenge,” Sant said. “Many of today’s approaches for carbon management either require more clean energy than we can produce or are unaffordable. As such, we need to create solutions that are accessible and that will not impoverish the world. We have tried to use a lens of pragmatism to consider how we may be able to achieve synthetic interventions at an unprecedented scale, while considering the finite energy and financial resources we have.”
Still, the researchers believe that sCS2, even at smaller scales, represents an advance in carbon-capture and storage that should be considered as a potential part of any overall strategy for confronting climate change.
Other co-authors of the study are UCLA’s Dante Simonetti, an assistant professor of chemical and biomolecular engineering; Jingbo Wang, a postdoctoral researcher; Abdulaziz Alturki, a Ph.D. graduate who is now an assistant professor at King Abdulaziz University in Saudi Arabia; Xin Chen, an associate development engineer; and David Jassby, an associate professor of civil and environmental engineering.
The research was supported by the U.S. Department of Energy’s Office of Fossil Energy, the Anthony and Jeanne Pritzker Family Foundation, the Grantham Foundation for the Protection of the Environment, the National Science Foundation, the U.S.–China Clean Energy Research Center for Water-Energy Technologies, the University of Texas at Arlington and the UCLA Institute for Carbon Management.


